Overview
The PlasmaBlade™ soft tissue dissection device uses brief, precise pulses of radiofrequency (RF) energy to cut and coagulate soft tissue. RF energy, combined with a proprietary insulation technology, enables the PlasmaBlade to dissect with the precision of a scalpel, and the bleeding control of traditional electrosurgery, while producing minimal thermal damage to surrounding tissue.
Clinical research has demonstrated that low thermal injury technology offers intra-operative and post-operative benefits when compared to the current standard of care — scalpel and traditional electrosurgery.1,2,3


Indications
The PlasmaBlade is indicated for cutting and coagulation of soft tissue during General, Plastic and Reconstruction (including but not limited to skin incisions and development of skin flaps), ENT, Gynecologic, Orthopaedic, Arthroscopic, Spinal and Neurological procedures.
Thermal Image Profile
TRADITIONAL
ELECTROSURGERY
Plasmablade
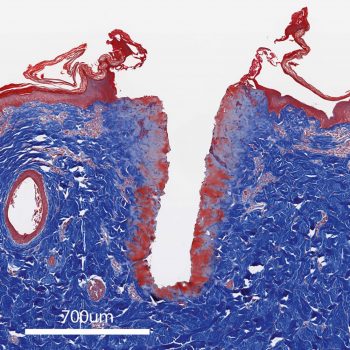
Incision using a traditional electrosurgical tool using the CUT 35W setting, showing significant thermal injury.
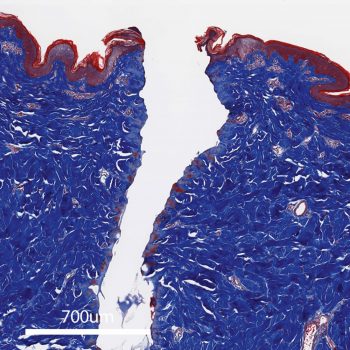
PlasmaBlade™ device incision using the CUT 6 setting, showing low thermal in jury.
These histologic profiles4 compare thermal injury (red) with PlasmaBlade and traditional electrosurgery devices at similar cut settings.
BENEFITS
LOWER TEMPERATURE
- 64% average reduction in blade temperature for similar CUT settings compared to traditional electrosurgery1
- 45% average reduction in blade temperature for similar COAG settings compared to traditional electrosurgery1
INCISION HEALING
- Equivalent cutaneous healing to scalpel,5,*
- Significantly reduced thermal injury depth and scar width compared to traditional electrosurgery1
INCREASED EFFICIENCY
- Maintains cutting effectiveness and hemostatic ability even when submerged in liquefied tissue or blood, unlike traditional electrosurgical tools7
REDUCED SURGICAL SMOKE
- Less surgical smoke8, allowing for increased visibility
REDUCED RISK
- Eliminates risk of inadvertent scalpel injury
Operating temperature profile5,*
Higher operating temperature is shown in infrared images.
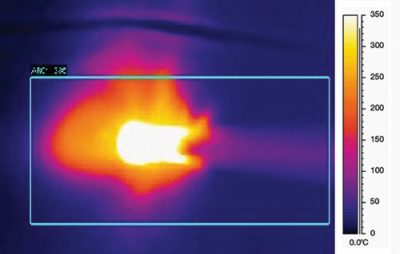
Traditional Electrosurgery: 200-350°C

Operating temperature profile of the PlasmaBlade™ device dissection device using Infrared imaging.
Surgical Applications
![]()
Ear, nose, and throat (ENT)
- Tonsillectomy
- Adenoidectomy
- Uvulopalatopharyngoplasty (UPPP)
![]()
Orthopaedics
- Total knee arthroplasty
- Total hip arthroplasty
![]()
Breast oncology
- Mastectomy
- Skin-sparing mastectomy
- Nipple-sparing mastectomy
- Oncoplasty
![]()
Cardiac implantable electronic devices
- Generator changeouts
- Generator replacements
- Generator upgrades
- Capsulectomy
![]()
Spine
- Anterior cervical discectomy infusion
- Multilevel spinal fusions
- Posterior cervical discectomy and fusion
- Posterior lumbar interbody fusion (PLIF)
- Transforaminal lumbar interbody fusion (TLIF)
- Anterior lumbar interbody fusion (ALIF)
- Minimally invasive TLIF
- Scoliosis surgery
- Laminotomy, discectomy, decompression
Model Features

AEX GENERATOR
Powers all Aquamantys and PlasmaBlade devices and provides simultaneous activation of both technologies.
- Touchscreen interface
- Four memory settings
- Lightweight
- Rapid startup and priming

PLASMABLADE 3.0S
- Ergonomic handle design for comfort and control
- Adjustable telescoping shaft
- Locking mechanism to secure shaft at desired length
- Integrated smoke evacuation

PLASMABLADE 4.0
- Ergonomic handle design for comfort and control
- Bendable shaft
- Rotating finger grip

PLASMABLADE NEEDLE
- Fine needlepoint tip
- Designed for ultra-precise surgical procedures and working of very delicate skin, such as the face and eyelids

PLASMABLADE PLUS
- 3.0mm wide blade for precision dissection
- Interchangeable CoagCap for enhanced bleeding control
- Integrated suction for improved visibility
- Ergonomic handle design for comfort and control

PLASMABLADE ENT FAMILY
- Four tip designs for tonsil, adenoid, and uvulopalatopharyngoplasty (UPPP) procedures
- Ergonomic handle design for comfort and control
- Bendable shaft
- Rotating finger grip

Senden Sie uns Ihre Anfrage
Wir helfen Ihnen gerne weiter bei der Produktberatung und machen Ihnen ein faires Angebot.
Rufen Sie uns gerne an: Tel.: 0511 18 383
oder senden Sie uns einfach eine unverbindliche Anfrage.
Ihr Team von ACENDIS Aesthetics

ACENDIS Aesthetics GmbH
Wohlenbergstr. 5
30179 Hannover
GERMANY
* Operating temperature is a function of device settings, electrode configuration and treatment time. Operating temperatures outside this range may be observed.




